As consumer demand for personalized health solutions rises, Vitamyna, a leading supplement company, launches its innovative Personal Pack Builder feature, revolutionizing how individuals address their nutritional and wellness needs. This technology allows customers to visit the vitamyna.com website and create custom daily vitamin packs with their chosen supplements, which are then prepared and conveniently delivered […]
Tag: Clinical Research
LyGenesis Announces First Patient Dosed in its Phase 2a Clinical Trial of a First-in-Class Regenerative Cell Therapy for Patients with End-Stage Liver Disease
LyGenesis, a clinical-stage biotechnology company developing cell therapies for large unmet medical needs, announced today that the first patient has been dosed in their Phase 2a clinical trial evaluating their first-in-class allogenic regenerative cell therapy transplanted into patients’ lymph nodes as a potential treatment for end-stage liver disease (ESLD). “In a medical first, we have […]
Ultimovacs announces publication of results from NIPU Phase II trial with UV1 vaccination in mesothelioma in European Journal of Cancer
Ultimovacs ASA (“Ultimovacs”) (OSE ULTI), a clinical-stage biotechnology company developing immunotherapeutic cancer vaccines, today announced that the results from the randomized controlled Phase II clinical trial, NIPU, are published in the European Journal of Cancer. The trial investigates the effect of adding Ultimovacs’ cancer vaccine UV1 to second-line treatment with ipilimumab and nivolumab for patients with […]
CG Oncology Announces First Patient Dosed in PIVOT-006 Phase 3 Clinical Trial of Cretostimogene in Intermediate-risk Non-Muscle Invasive Bladder Cancer
CG Oncology, Inc. (NASDAQ: CGON), a late-stage clinical biopharmaceutical company focused on developing and commercializing a potential backbone bladder-sparing therapeutic for patients afflicted with bladder cancer, today announced the first patient has been dosed in the PIVOT-006 Phase 3 clinical trial of cretostimogene for the treatment of patients with intermediate-risk NMIBC following transurethral resection of […]
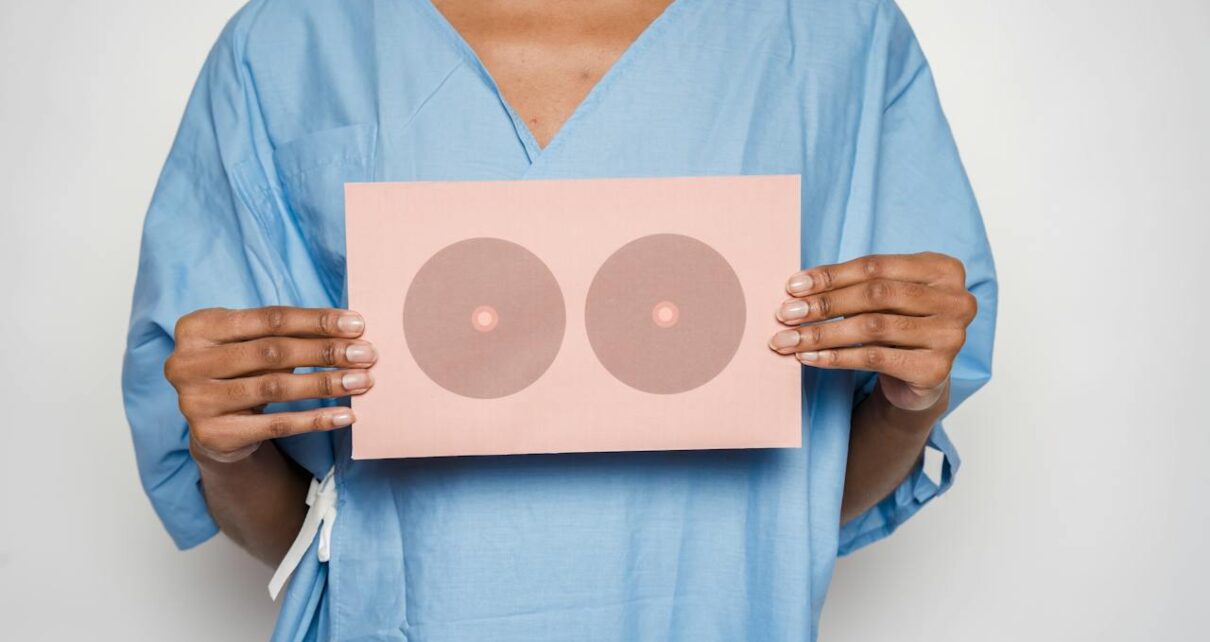
New Guidance Published in The Lancet Recommends Early Intervention and Use of BIS to Reduce Impact of Arm Lymphedema on Breast Cancer Patients
ImpediMed, a pioneer in the field of medical technology, announced a significant advancement in the fight against breast cancer-related lymphedema, a common but often overlooked condition affecting breast cancer survivors. Leading medical journal eClinicalMedicine, part of The Lancet Discovery Science, recently published new clinical guidance advocating for early detection and prevention strategies, underscoring the validity and efficacy […]
Wasatch Biolabs Launches Proprietary Targeted DNA Methylation Sequencing Service for Researchers and Healthcare Providers
Wasatch Biolabs (WBL), a subsidiary of Renew Biotechnologies and a certified Oxford Nanopore Technologies’ laboratory, launches a proprietary Targeted DNA Methylation Sequencing Service for researchers and clinical service providers. The technique is amplification and bisulfite conversion free, effectively bypassing the most common limitations of targeted methylation analysis and enabling WBL to create custom methylation panels that target […]
Hemab Therapeutics Presents Positive Phase 1 Results for HMB-001 in Glanzmann Thrombasthenia at 2024 EAHAD Annual Congress
Hemab Therapeutics, a clinical-stage biotechnology company developing novel prophylactic therapeutics for serious, underserved bleeding and thrombotic disorders, today presented initial results from Phase 1 of its ongoing evaluation of HMB-001. A novel bispecific antibody, HMB-001 is in development as a prophylactic treatment for the bleeding disorder Glanzmann Thrombasthenia (Glanzmann). The data were presented as one of […]
ProLynx announces initiation of Phase I/II clinical trial of its DNA-damaging agent PLX038 in patients with rare CNS tumors at the National Cancer Institute (NCI)
ProLynx Inc. announced today that the first patient was treated with PLX038 (PEGylated SN-38) in a Phase I/II clinical trial for primary CNS tumors driven by MYC or MYCN amplifications. National Institutes of Health’s NCI investigators Dr. Marta Penas-Prado and Dr. Mark Gilbert are conducting the trial. MYC genes regulate expression of genes involved in cell division. High levels of […]
Constant Therapeutics Announces First Patient Dosed in Phase 2 Clinical Trial of TXA127 in Ischemic Stroke Recovery
Constant Therapeutics LLC, a biopharmaceutical company focused on the development of treatments impacting the Alternative Renin-Angiotensin System, today announced that the first patient has been dosed in the Company’s phase 2 clinical trial of TXA127, the Company’s lead peptide product under development as a potential treatment for ischemic stroke recovery. The phase 2 trial is a […]
Lynk Pharmaceuticals Announced First Rheumatoid Arthritis Patient Dosed in Phase Ⅲ Clinical Study of LNK01001
Lynk Pharmaceuticals Co., Ltd. (hereinafter referred to as ‘Lynk Pharmaceuticals’), an innovative clinical stage company, announced that it has dosed the first patient with Rheumatoid Arthritis (RA) in a Phase Ⅲ clinical trial of its highly selective JAK1 inhibitor LNK01001. The clinical study is a randomized, double-blind, placebo-controlled Phase III trial evaluating the efficacy and […]