Constant Therapeutics LLC, a biopharmaceutical company focused on the development of treatments impacting the Alternative Renin-Angiotensin System, today announced that the first patient has been dosed in the Company’s phase 2 clinical trial of TXA127, the Company’s lead peptide product under development as a potential treatment for ischemic stroke recovery. The phase 2 trial is a […]
Tag: medical breakthrough
Enhancing Leadership in Gynecology, Asieris Pharmaceuticals Appoints Sophia Cao to Lead the Newly-Established Women’s Health Business Unit, Accelerating Strategic Expansion
Asieris Pharmaceuticals (Stock code :688176.SH) has announced its set-up of the Women’s Health Business Unit for commercialization. This strategic move is designed to enhance the company’s focus on genitourinary diseases and strengthen its position in women’s health. The new business unit will center around core asset APL-1702, a potentially first-in-class non-surgical treatment of cervical high-grade squamous […]
FDA Approves First Vaccine to Prevent Disease Caused by Chikungunya Virus
Today, the U.S. Food and Drug Administration approved Ixchiq, the first chikungunya vaccine. Ixchiq is approved for individuals 18 years of age and older who are at increased risk of exposure to chikungunya virus. The chikungunya virus is primarily transmitted to people through the bite of an infected mosquito. Chikungunya is an emerging global health threat […]
The World’s First “Recombinant Anthrax Vaccine”: GC Biopharma applies for MFDS Approval
GC Biopharma announced 1 Nov. 2023 that it has applied for the marketing approval of “GC1109”, an anthrax vaccine jointly developed by GC Biopharma and the Korea Disease Control and Prevention Agency (KDCA), to the Korean Ministry of Food and Drug Safety (MFDS). ‘GC1109’ contains protective antigen (PA) as its active pharmaceutical ingredients produced by recombinant DNA […]
First Results with Erdafitinib-Releasing Intravesical Delivery System (TAR-210) Show Early Evidence of Positive Clinical Activity in Patients with Non-Muscle-Invasive Bladder Cancer with Select Fibroblast Growth Factor Receptor Alterations
The Janssen Pharmaceutical Companies of Johnson & Johnson announced today the first results from an open-label, multicenter Phase 1 study evaluating the safety and efficacy of TAR-210, an intravesical delivery system designed to provide sustained, local release of erdafitinib into the bladder in patients with non-muscle-invasive bladder cancer (NMIBC) with select fibroblast growth factor receptor (FGFR) […]
AMGEN PRESENTS NEW LUMAKRAS® (SOTORASIB) PLUS VECTIBIX® (PANITUMUMAB) DATA IN PATIENTS WITH KRAS G12C-MUTATED METASTATIC COLORECTAL CANCER
Amgen (NASDAQ:AMGN) today announced data from the global Phase 3 CodeBreaK 300 trial evaluating two doses of LUMAKRAS® (sotorasib) (960 mg or 240 mg) in combination with Vectibix® (panitumumab). Both doses demonstrated a statistically significant superiority in progression-free survival (PFS) over the investigator’s choice of therapy in patients with chemorefractory KRAS G12C-mutated metastatic colorectal cancer (mCRC). The results are being presented today […]
Avidity Biosciences Announces New Positive AOC 1001 Data Demonstrating Improvement in Multiple Additional Functional Endpoints and Favorable Long-term Safety and Tolerability in People with Myotonic Dystrophy Type 1
Avidity Biosciences, Inc. (Nasdaq: RNA), a biopharmaceutical company committed to delivering a new class of RNA therapeutics called Antibody Oligonucleotide Conjugates (AOCs™), today announced new positive AOC 1001 data demonstrating improvement in multiple additional functional endpoints and favorable long-term safety and tolerability in people living with myotonic dystrophy type 1 (DM1). AOC 1001, Avidity’s lead clinical […]
Via Nova Therapeutics Announces FDA clearance of Investigational New Drug (IND) application for VNT-101, a novel direct-acting antiviral against Influenza A virus
Via Nova Therapeutics, Inc., a biotechnology company focused on discovering and developing therapeutics to treat viral infections with significant unmet medical need, today announced the clearance of its Investigational New Drug (IND) application from the U.S. Food and Drug Administration (FDA) for its Influenza A nucleoprotein inhibitor, VNT-101. “We look forward to evaluating VNT-101 in […]
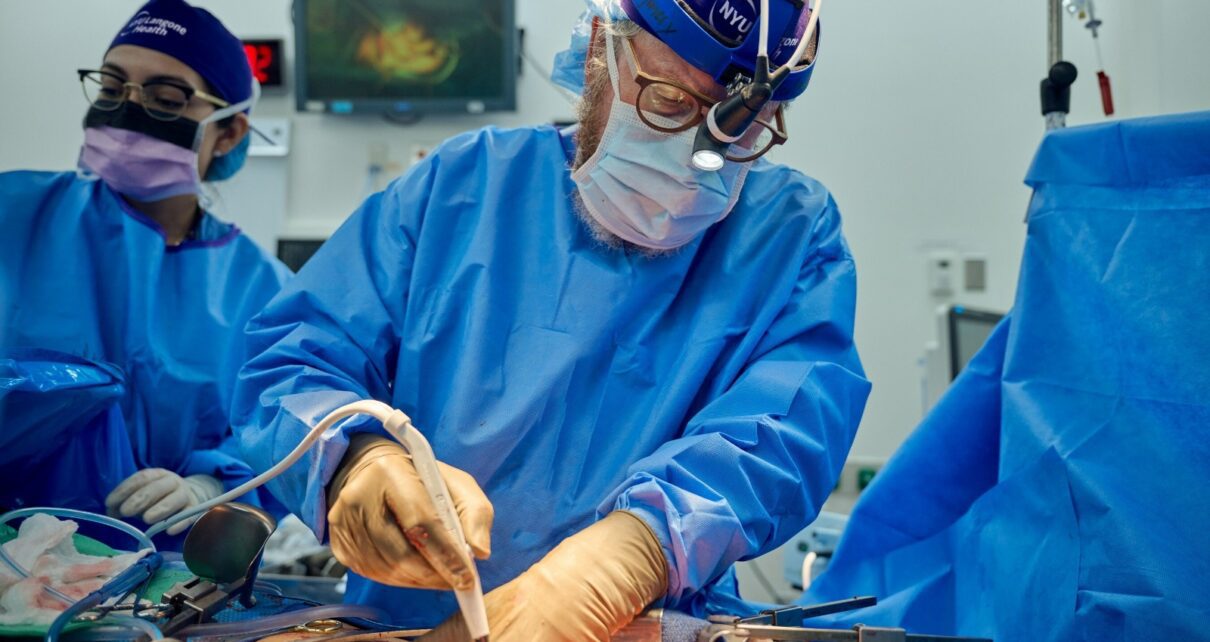
Two-Month Study of Pig Kidney Xenotransplantation Gives New Hope to the Future of the Organ Supply
After 61 days of observation, NYU Langone Health doctors this month completed the longest-documented case of a genetically engineered pig kidney functioning in a human body. The procedure, known as a xenotransplant, which involves the transplant of an animal organ into a human, was performed on July 14, 2023, and led by Robert Montgomery, MD, […]
Paras Health Gurugram achieves the milestone of becoming the 3rd Centre in India to successfully utilize the latest C2 plus IVL Catheter Technology for Cardiology Complex Angioplasty
Paras Health Gurugram, a pioneer in advanced medical care, has achieved another remarkable technological milestone by becoming the third medical center in India to adopt the groundbreaking C2 Plus IVL Catheter technology within five days of its launch in India. The Director & Unit Head, Interventional Cardiology at Paras Health, Dr. Amit Bhushan Sharma, successfully […]